| Section | Summary |
|---|---|
| Role of Internal Audits in ISO 9001 | Explains the significance of internal audits within the ISO 9001 framework and their role in the Plan-Do-Check-Act cycle. |
| Preparing for an ISO 9001 Internal Audit | Step-by-step guide to preparing for an internal audit, including defining objectives, selecting the audit team, and developing a schedule. |
| Conducting the ISO 9001 Internal Audit | Details the process of conducting an audit, including opening meetings, audit techniques, and identifying non-conformities. |
| Post ISO 9001 Audit Activities | Describes the actions to take after the audit, such as preparing the audit report, discussing findings, and developing corrective action plans. |
| Conclusion | Summarizes the importance of internal audits in maintaining and improving a robust quality management system. |
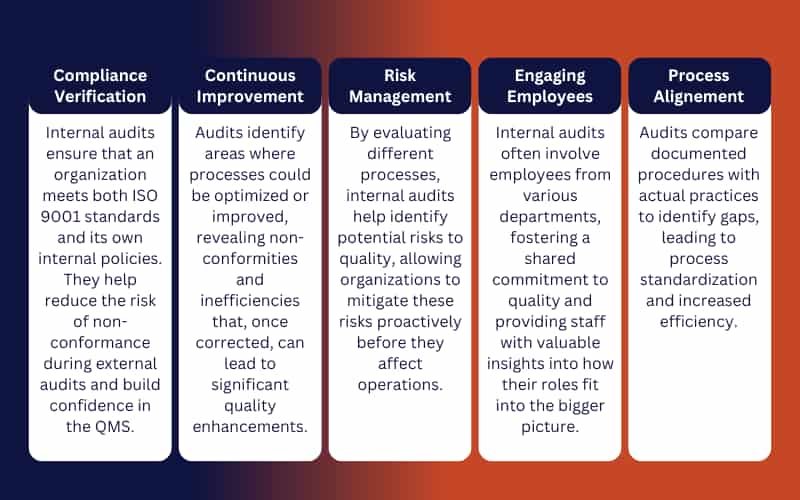
ISO 9001 internal audit is integral to maintaining a high standard of quality management within an organization. It is an essential step in ensuring that a company adheres to the guidelines and requirements set out in the ISO 9001 standard. By conducting regular internal audits, organizations can assess their quality management system (QMS), uncovering opportunities for improvement while ensuring compliance with international standards.
The ISO 9001 standard is globally recognized as a framework for developing and managing a QMS. It encourages organizations to adopt several quality management principles, such as strong customer focus, leadership involvement, and continual improvement. The standard provides a structured approach to quality, guiding organizations in implementing processes that consistently deliver products and services that meet both customer and regulatory requirements. Internal audits are crucial in verifying that the QMS remains compliant and effective in this regard.
Conducting internal audits offers a range of benefits that go beyond mere compliance:
I. Role of Internal Audits in ISO 9001
Internal audits form an integral part of ISO 9001’s “Plan-Do-Check-Act” cycle. They serve as the “Check” stage, ensuring that the implemented processes (the “Do” stage) align with the planned goals and comply with the quality policy and objectives. Internal audits are also a feedback mechanism for the “Act” stage, providing data that can be used to adjust the QMS and refine strategies for improvement.
Audits are designed to be systematic and independent, evaluating all aspects of the QMS, from leadership and documentation to manufacturing and customer satisfaction. A well-planned and executed internal audit is instrumental in verifying that the organization remains compliant with ISO 9001 and continues to meet its quality objectives effectively.
Ultimately, ISO 9001 internal audits offer organizations the tools and insights they need to continually improve their QMS, ensuring consistent product and service quality while fostering a culture of excellence.
II. Preparing for an ISO 9001 Internal Audit
Preparation is the foundation of a successful ISO 9001 internal audit. A well-prepared audit helps ensure a smooth process, provides valuable insights, and sets the stage for effective improvements. Here’s a step-by-step guide to prepare for the internal audit:
A – Understanding Audit Objectives and Criteria
Start by clarifying the purpose and scope of the audit. This involves defining the objectives, such as verifying compliance with ISO 9001 requirements, evaluating internal policies, or identifying areas for improvement. Establishing clear criteria ensures that the audit will address key performance indicators and standards relevant to your quality management system (QMS).
To determine the scope, identify the specific processes, departments, or functions that will be audited. This step should be based on factors like risk assessments, past audit findings, or management concerns. A well-defined scope prevents the audit from becoming too broad or unfocused.
B – Selecting the Audit Team
The effectiveness of an internal audit largely depends on the competence and impartiality of the audit team. Choose team members who are knowledgeable about ISO 9001 requirements and have a thorough understanding of your organization’s processes. To ensure objectivity, auditors should not assess their own departments or responsibilities. Consider providing additional training or certification to enhance their auditing skills, especially if they’re new to the role.
C- Developing an ISO 9001 Internal Audit Schedule
An organized audit schedule helps to streamline the entire process. Develop a timeline that outlines when each phase of the audit will occur, and which departments will be involved. This allows both the audit team and auditees to prepare accordingly.
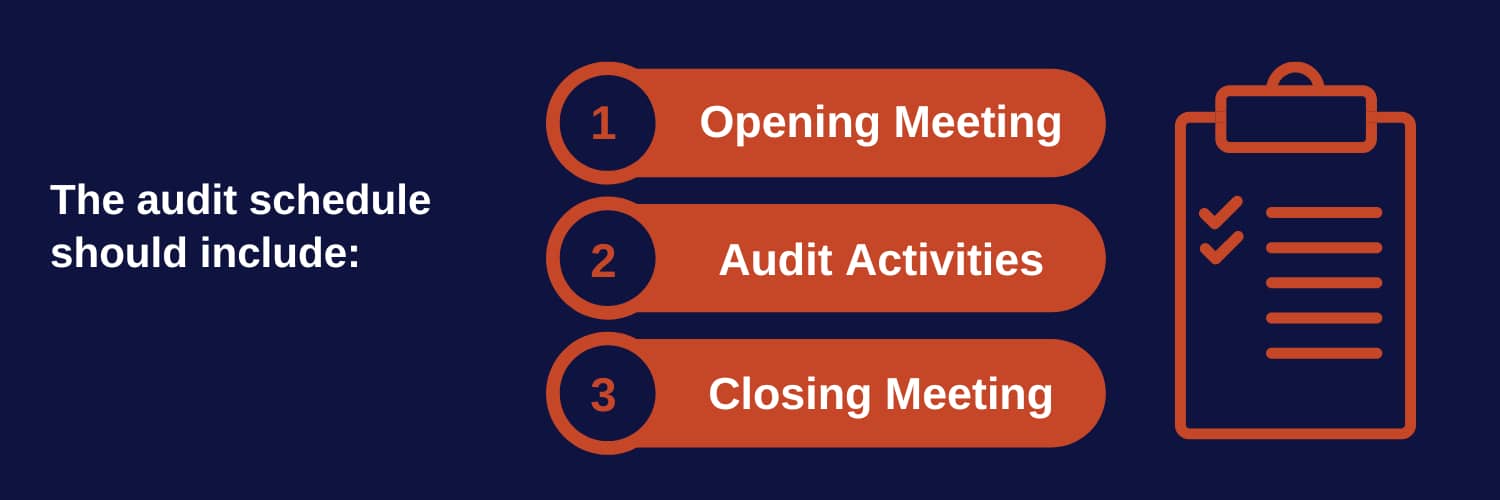
- Opening Meeting: An initial briefing to introduce the audit team and explain the audit’s purpose.
- Audit Activities: Allocate time for document reviews, interviews, and process observations.
- Closing Meeting: A final meeting to discuss findings and provide initial feedback.
Share the schedule with relevant departments to ensure key personnel are available during the audit.
D – Preparing Audit Documentation
Collect and review all relevant documents, such as quality manuals, work instructions, and previous audit reports. This provides a background on the processes to be audited and any past issues that might need re-evaluation. Develop checklists based on the audit criteria to help guide the team through the assessment and ensure no critical aspects are overlooked.
E – Communicating with Departments
Effective communication is key to a successful audit. Notify department heads and other key personnel about the upcoming audit, explaining its purpose and how they can best prepare. Emphasize that the audit is a collaborative effort aimed at improving the QMS, not a punitive process. Encourage open dialogue and let them know what to expect in terms of interviews, document requests, and observations.
F – Final Review of the ISO 9001 Internal Audit Plan
Before commencing the audit, conduct a final review of the plan with the audit team. Make sure everyone understands their roles, the audit criteria, and the schedule. Adjust the plan as needed to accommodate emerging priorities or department concerns. A well-reviewed plan provides the roadmap necessary for an effective and efficient internal audit.
III. Conducting the ISO 9001 Internal Audit
Conducting an ISO 9001 internal audit requires meticulous planning, attention to detail, and effective communication. The purpose is to provide a comprehensive assessment of your quality management system (QMS), ensuring it meets both ISO standards and internal quality objectives.
A – Opening Meeting
Begin with an opening meeting that brings together the audit team and key personnel from the departments being audited. The purpose of this meeting is to clarify the audit’s objectives, explain the agenda, and set expectations. Discuss the scope, methodology, and criteria to be used during the audit. Encourage participants to ask questions and share any immediate concerns. This meeting ensures everyone understands the process and feels comfortable cooperating with the audit team.
B – ISO 9001 Internal Audit Execution Techniques
When performing the audit, use a mix of techniques to collect accurate and comprehensive data. These include:
- Interviewing Staff: Conduct interviews with relevant staff members to understand their roles and how they contribute to the QMS. Ask open-ended questions that invite detailed responses and encourage them to describe their tasks, challenges, and compliance with documented procedures. Interviews reveal the practical application of policies and help identify discrepancies between documented and actual practices.
- Reviewing Documents and Records: Examine essential documents, such as quality manuals, procedures, and work instructions, to verify compliance with ISO 9001 standards. Check that records are accurate, up-to-date, and maintained according to the established procedures. This review provides evidence of effective process control, traceability, and adherence to requirements.
- Observing Processes: Observe processes in action to validate that they align with documented procedures. This could include witnessing production lines, reviewing testing protocols, or monitoring customer service activities. Observing processes helps detect potential non-conformities or areas for improvement that might not be immediately evident through documentation alone.
C – Identifying Non-Conformities
During the audit, document any findings that indicate non-conformities, inefficiencies, or deviations from standards. Classify these findings based on their severity and potential impact on quality. Minor non-conformities might represent small procedural issues, while major ones could signal a significant lapse in compliance that requires immediate action. Be objective in your assessment and provide clear evidence to support each finding.
IV. Post ISO 9001 Audit Activities
The post-audit phase is crucial to translating audit findings into meaningful actions that enhance the quality management system (QMS). This stage involves preparing a comprehensive report, communicating the results to stakeholders, and collaborating with management and staff to implement improvements.
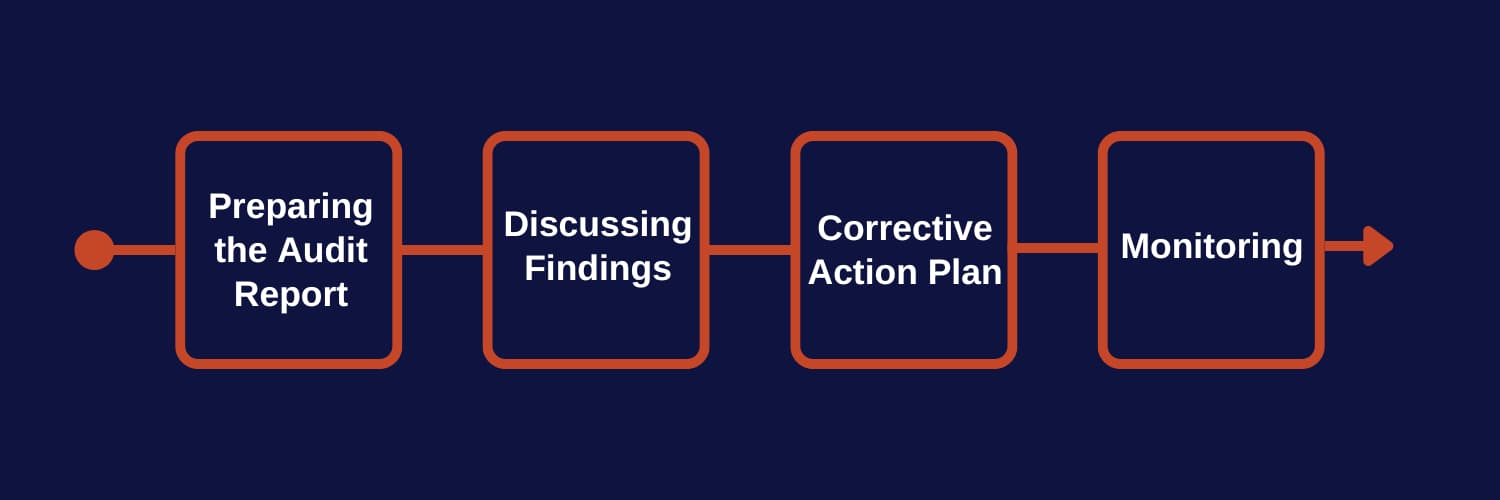
A – Preparing the ISO 9001 Audit Report
The audit report is a formal record of the audit’s findings and serves as a basis for planning corrective actions. It should be clear, concise, and organized to help management prioritize areas for improvement. Include the following components:
- Introduction: State the purpose, scope, and objectives of the audit.
- Summary of Findings: Provide an overview of the main non-conformities, observations, and positive practices identified.
- Detailed Findings: Present each non-conformity with supporting evidence, classification (e.g., minor, or major), and references to relevant standards or procedures.
- Recommendations: Suggest corrective actions or areas that require further analysis to address non-conformities.
Make sure the report uses straightforward language and clearly distinguishes between recommendations and mandatory corrective actions.
B – Discussing Findings and Recommendations
After the report is finalized, share it with the relevant stakeholders, including department heads, quality managers, and senior leadership. Schedule a follow-up meeting to discuss the findings and answer questions. This meeting should focus on understanding the root causes of non-conformities and setting priorities for corrective actions. Collaborative discussions help ensure that everyone involved is committed to making the necessary improvements and understands their role in the corrective action process.
C – Developing a Corrective Action Plan
Based on the audit report, develop a corrective action plan to address the identified non-conformities and prevent their recurrence. This plan should include:
- Actions to Be Taken: Clearly outline what corrective measures will be implemented.
- Responsible Parties: Assign responsibility to specific individuals or departments.
- Timeline: Set deadlines for each corrective action.
- Verification: Define how the effectiveness of each corrective action will be verified.
Make sure that corrective actions align with the audit recommendations and are realistic given available resources.
D – Monitoring Implementation
Once the corrective action plan is in place, monitor its progress to ensure that deadlines are met and improvements are effective. Regularly update management on the status of each action item. If challenges arise, work with the relevant departments to address them promptly and adjust the plan if necessary. Effective monitoring ensures that the post-audit improvements are implemented as intended and sustain long-term quality gains.
This monitoring can be done through:
- Regular Check-Ins: Schedule periodic reviews to assess the status and effectiveness of each corrective action.
- Performance Indicators: Use specific metrics or indicators to measure improvements. For example, if a corrective action was aimed at reducing product defects, track defect rates before and after the implementation to gauge effectiveness.
- Feedback Collection: Gather feedback from employees directly affected by the changes. Their insights can indicate how well the improvements are working and whether further adjustments are needed.
E – Closing non-conformities
For each non-conformity addressed, determine if the corrective action has fully resolved the issue:
- Verification Audits: Conduct targeted audits to verify that the non-conformities have been effectively addressed. These can be part of regular internal audits or special follow-up audits.
- Closing Out Non-Conformities: If a non-conformity is resolved, formally close it out in the QMS records. Document the resolution process and the outcomes to maintain a clear record of quality improvements.
F – Planning the Next ISO 9001 Internal Audit Cycle
Each audit provides valuable insights that should be used to shape future audits. When planning the next cycle, reassess the frequency of internal audits based on past findings and current business needs. If the last audit cycle revealed significant non-conformities or systemic issues, more frequent audits may be necessary. Use lessons learned from previous audits to refine the scope and focus of future ones, prioritizing high-risk areas or processes that underwent significant changes since the last audit. Strengthen the audit team’s expertise by providing training or certification. A well-prepared team ensures more accurate and actionable findings.

V. Conclusion
The practice of conducting ISO 9001 internal audits is an essential component of maintaining a robust quality management system (QMS). These audits provide an invaluable mechanism for organizations to ensure compliance with ISO standards, identify areas for improvement, and foster a culture of continuous quality enhancement. This detailed examination has walked through the crucial phases of preparing for, conducting, and following up on internal audits, as well as planning for continual improvement.


